Lab 21 organic compounds alkanes – Embark on a captivating journey into the realm of organic chemistry as we delve into Lab 21: Organic Compounds – Alkanes. This comprehensive exploration will unravel the structural intricacies, nomenclature, physical and chemical properties, and industrial applications of these fundamental hydrocarbons, providing a solid foundation for further understanding in this fascinating field.
Alkanes, the simplest and most abundant class of organic compounds, form the backbone of fuels, petrochemicals, and countless everyday products. Their unique characteristics, governed by their molecular structure, dictate their behavior and utility. Join us as we uncover the secrets of these versatile molecules, gaining insights into their role in shaping our world.
Definition of Alkanes

Alkanes are a class of organic compounds that consist solely of carbon and hydrogen atoms, and are characterized by a structure composed entirely of single bonds. These compounds are acyclic, meaning they do not contain any rings in their molecular structure.
The general formula for alkanes is CnH2n+2, where n represents the number of carbon atoms in the molecule. Alkanes can be linear or branched, and the number of possible isomers increases rapidly with the number of carbon atoms.
Structural Formula of Alkanes
The structural formula of alkanes can be represented as a straight chain of carbon atoms with hydrogen atoms attached to each carbon. The carbon atoms are connected by single bonds, and the hydrogen atoms are connected to the carbon atoms by single bonds as well.
Examples of Alkanes
- Methane (CH4)
- Ethane (C2H6)
- Propane (C3H8)
- Butane (C4H10)
- Pentane (C5H12)
General Properties of Alkanes
- Alkanes are generally nonpolar and unreactive.
- They are insoluble in water but soluble in organic solvents.
- They have low boiling points and melting points.
- They are colorless and odorless.
Nomenclature of Alkanes
Alkanes are a class of organic compounds that consist solely of carbon and hydrogen atoms. They are characterized by their single bonds between carbon atoms, forming an open chain or branched structure. The nomenclature of alkanes follows specific rules established by the International Union of Pure and Applied Chemistry (IUPAC).
IUPAC Rules for Naming Alkanes
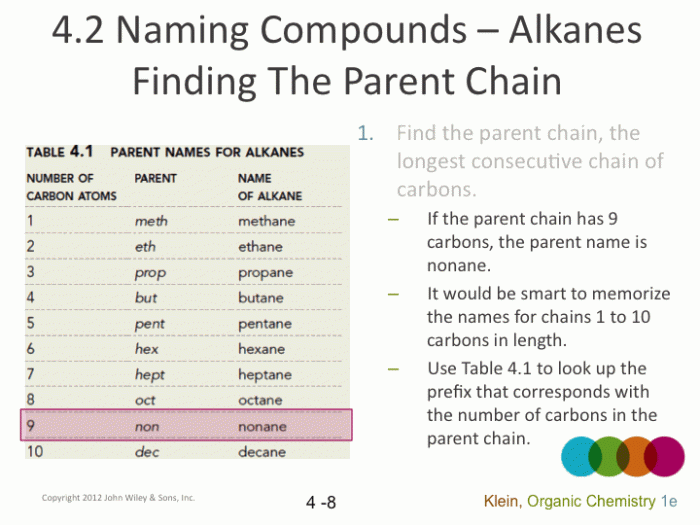
- Identify the parent chain:The parent chain is the longest continuous chain of carbon atoms in the molecule.
- Assign a root name:The root name is determined by the number of carbon atoms in the parent chain. (See table below for root names)
- Number the carbon atoms:Number the carbon atoms in the parent chain from one end to the other, starting with the end that gives the substituents the lowest numbers.
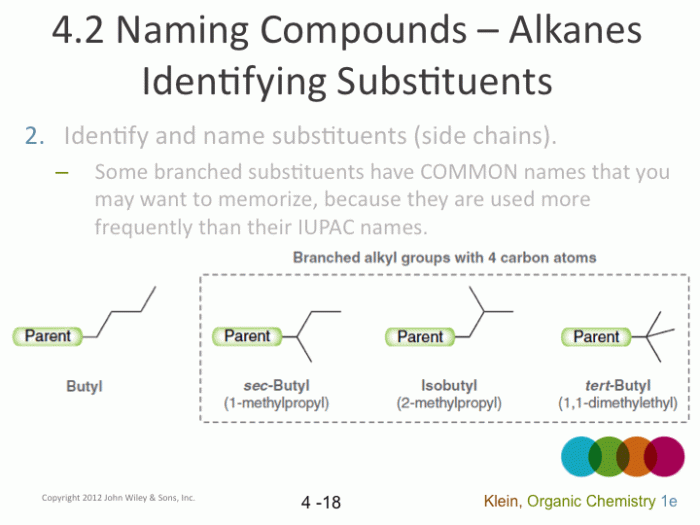
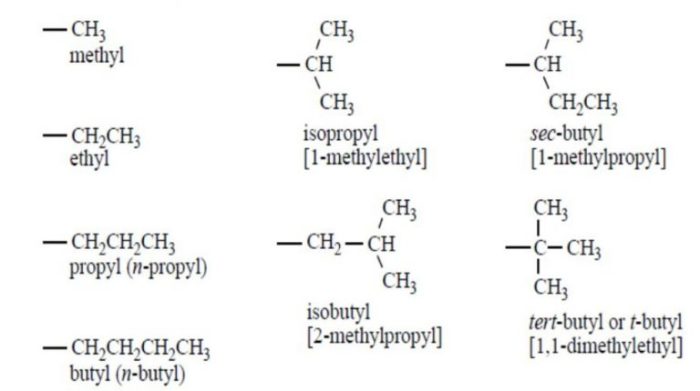
- Identify and name substituents:Substituents are groups of atoms attached to the parent chain. They are named according to the type of group (e.g., methyl, ethyl, etc.) and the carbon atom they are attached to.
- Combine the root name, substituents, and numbers:Combine the root name, the names of the substituents, and the numbers indicating their positions to form the complete name of the alkane.
Table of Alkane Names and Structural Formulas
| Root Name | Number of Carbon Atoms | Structural Formula |
|---|---|---|
| Meth- | 1 | CH4 |
| Eth- | 2 | C2H6 |
| Prop- | 3 | C3H8 |
| But- | 4 | C4H10 |
| Pent- | 5 | C5H12 |
| Hex- | 6 | C6H14 |
| Hept- | 7 | C7H16 |
| Oct- | 8 | C8H18 |
| Non- | 9 | C9H20 |
| Dec- | 10 | C10H22 |
Assigning Prefixes to Alkanes
The prefixes used in alkane nomenclature indicate the number of carbon atoms in the parent chain. The prefixes are as follows:
- Meth-: 1 carbon atom
- Eth-: 2 carbon atoms
- Prop-: 3 carbon atoms
- But-: 4 carbon atoms
- Pent-: 5 carbon atoms
- Hex-: 6 carbon atoms
- Hept-: 7 carbon atoms
- Oct-: 8 carbon atoms
- Non-: 9 carbon atoms
- Dec-: 10 carbon atoms
Physical Properties of Alkanes
Alkanes, being nonpolar and having relatively weak intermolecular forces (van der Waals forces), exhibit distinct physical properties that are directly influenced by their molecular weight.
Melting Points and Boiling Points
As the molecular weight of alkanes increases, so do their melting points and boiling points. This trend is attributed to the stronger intermolecular forces present in heavier alkanes. Heavier alkanes have a greater number of electrons, resulting in a larger surface area for van der Waals interactions.
| Name | Molecular Formula | Molecular Weight (g/mol) | Melting Point (°C) | Boiling Point (°C) |
|---|---|---|---|---|
| Methane | CH4 | 16.04 | -182.5 | -161.6 |
| Ethane | C2H6 | 30.07 | -183.3 | -88.6 |
| Propane | C3H8 | 44.10 | -189.7 | -42.1 |
| Butane | C4H10 | 58.12 | -138.3 | -0.5 |
| Pentane | C5H12 | 72.15 | -129.8 | 36.1 |
| Hexane | C6H14 | 86.18 | -95.3 | 68.7 |
| Heptane | C7H16 | 100.21 | -90.6 | 98.4 |
| Octane | C8H18 | 114.24 | -56.8 | 125.7 |
| Nonane | C9H20 | 128.27 | -53.5 | 150.8 |
| Decane | C10H22 | 142.29 | -29.7 | 174.0 |
Densities
Alkanes are generally less dense than water. As the molecular weight of alkanes increases, their densities also increase. This is because heavier alkanes have a larger number of atoms packed into a smaller volume, resulting in a higher density.
Chemical Properties of Alkanes
Alkanes are generally unreactive due to the strength of their C-C and C-H bonds. They do not undergo most common reactions, such as addition, electrophilic aromatic substitution, or nucleophilic substitution. However, they can undergo certain reactions, including combustion, halogenation, and cracking.
Combustion, Lab 21 organic compounds alkanes
Alkanes undergo combustion in the presence of oxygen to produce carbon dioxide and water. The reaction is highly exothermic, releasing a large amount of heat. The combustion of alkanes is the basis of many fuels, such as natural gas, propane, and gasoline.
CH4+ 2O 2→ CO 2+ 2H 2O + heat
Halogenation
Alkanes can undergo halogenation reactions with halogens (F 2, Cl 2, Br 2, or I 2) in the presence of light or heat. In this reaction, a hydrogen atom on the alkane is replaced by a halogen atom. The reaction is typically initiated by the formation of a free radical, which then reacts with the halogen molecule.
CH4+ Cl 2→ CH 3Cl + HCl
Cracking
Alkanes can undergo cracking reactions in the presence of heat and a catalyst. In this reaction, the alkane is broken down into smaller alkanes or alkenes. Cracking is an important process in the petroleum industry, as it allows for the production of gasoline and other fuels from heavier fractions of crude oil.
C16H 34→ C 8H 18+ C 8H 16
Uses of Alkanes: Lab 21 Organic Compounds Alkanes
Alkanes, as a class of saturated hydrocarbons, find diverse applications in modern industries. Their inherent properties and ease of chemical modification make them valuable feedstocks for a range of products.
Major Industrial Uses
- Fuels:Alkanes, particularly methane, propane, and butane, are widely used as fuels for heating, cooking, and transportation. Their high energy content and relatively clean combustion make them efficient and environmentally friendly fuel sources.
- Feedstocks for Petrochemicals:Alkanes serve as the primary feedstocks for the petrochemical industry. They undergo various chemical transformations, such as cracking, reforming, and polymerization, to produce a vast array of products, including plastics, synthetic fibers, and solvents.
Examples of Products Derived from Alkanes
- Polyethylene:Derived from ethylene, a simple alkene obtained from alkanes, polyethylene is a versatile plastic used in packaging, construction, and various consumer products.
- Polypropylene:Obtained from propylene, another alkene derived from alkanes, polypropylene is a lightweight and durable plastic widely used in automotive parts, appliances, and medical devices.
- Synthetic Fibers:Alkanes are used to produce synthetic fibers such as nylon and polyester. These fibers are strong, durable, and resistant to wrinkles, making them suitable for clothing, carpets, and industrial applications.
Answers to Common Questions
What are the key structural features of alkanes?
Alkanes are characterized by their saturated hydrocarbon chains, meaning each carbon atom is bonded to four other atoms, forming a tetrahedral geometry. They lack any double or triple bonds, resulting in a stable and unreactive molecular structure.
How are alkanes named according to IUPAC rules?
The IUPAC (International Union of Pure and Applied Chemistry) system assigns names to alkanes based on the number of carbon atoms in the chain. The root name is derived from the Greek numerical prefix indicating the number of carbons, followed by the suffix “-ane” to denote an alkane.
What is the relationship between the molecular weight of alkanes and their physical properties?
As the molecular weight of alkanes increases, their physical properties change. Higher molecular weight alkanes have stronger intermolecular forces, resulting in higher melting points, boiling points, and densities. This trend is observed as the number of carbon atoms in the chain increases.