The molecular and empirical formula worksheet is an essential tool for students learning about the fundamental concepts of chemistry. This comprehensive guide provides a thorough overview of molecular and empirical formulas, including their definitions, methods for determining them, and their significance in the field of chemistry.
This guide delves into the intricacies of molecular and empirical formulas, explaining the differences between the two and how to calculate each type. It also includes practice problems and discussion questions to reinforce understanding.
Molecular and Empirical Formula Worksheet

This worksheet provides practice problems for determining the molecular and empirical formulas of compounds. It also includes a discussion of the importance of understanding these formulas and how they are used in chemistry.
Molecular Formula, Molecular and empirical formula worksheet
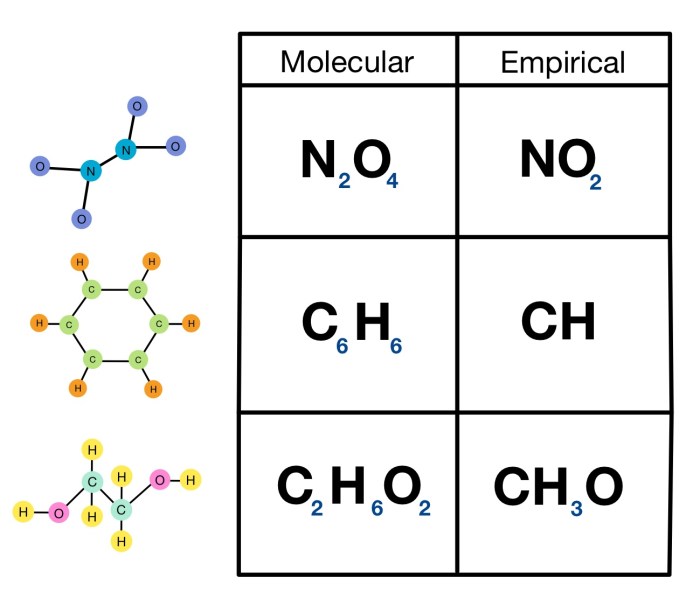
A molecular formula represents the exact number and type of atoms present in a molecule of a compound. For example, the molecular formula of water is H 2O, indicating that each molecule of water contains two hydrogen atoms and one oxygen atom.
Empirical Formula
An empirical formula represents the simplest whole-number ratio of atoms in a compound. It does not necessarily represent the actual number of atoms in a molecule. For example, the empirical formula of glucose is CH 2O, indicating that glucose contains carbon, hydrogen, and oxygen in a 1:2:1 ratio.
Worksheet Activities
- Problem 1:Determine the molecular formula of a compound that contains 40% carbon, 6.7% hydrogen, and 53.3% oxygen.
- Problem 2:Calculate the empirical formula of a compound that contains 24.3% sodium, 17.1% carbon, and 58.6% chlorine.
Examples and Methods
- Example 1:To determine the molecular formula of a compound with 40% carbon, 6.7% hydrogen, and 53.3% oxygen, we can use the following steps:
- Convert the percentages to grams.
- Convert the grams to moles.
- Divide the moles of each element by the smallest number of moles.
- Multiply the resulting ratios by the appropriate whole numbers to obtain the simplest whole-number ratio.
- Example 2:To calculate the empirical formula of a compound with 24.3% sodium, 17.1% carbon, and 58.6% chlorine, we can use the following steps:
- Convert the percentages to grams.
- Convert the grams to moles.
- Divide the moles of each element by the smallest number of moles.
Discussion Questions
- Why is it important to understand molecular and empirical formulas?
- How are molecular and empirical formulas used in chemistry?
Questions Often Asked
What is the difference between a molecular formula and an empirical formula?
A molecular formula represents the exact number and type of atoms in a molecule, while an empirical formula represents the simplest whole-number ratio of atoms in a compound.
How do I calculate the molecular formula of a compound?
To calculate the molecular formula, you need to know the empirical formula and the molar mass of the compound.
What is the significance of molecular and empirical formulas in chemistry?
Molecular and empirical formulas are essential for understanding the structure and composition of compounds. They are used in stoichiometry, chemical reactions, and various other chemical calculations.